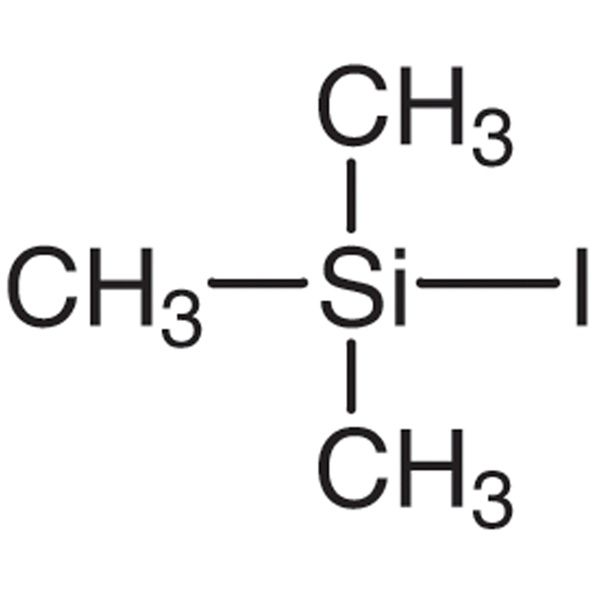
Chemical Properties:
Package: Fluorinated Bottle, 25kg/Drum, or according to customer's requirement Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moistureShanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Iodotrimethylsilane (CAS: 16029-98-4) with high quality. We can provide COA, worldwide delivery, small and bulk quantities available. Please contact: alvin@ruifuchem.com| Item | Specifications |
| Appearance | Colorless to Off-Red Liquid |
| Purity / Analysis Method | >99.0% (Argentmetric Titration) |
| Refractive Index n20/D | 1.47~1.48 |
| Stabilizer (Copper Chip) | Conforms |
| Infrared Spectrum | Conforms to Structure |
| Proton NMR Spectrum | Conforms to Structure |
| Test Standard | Enterprise Standard |
Description:
Specifications:
Package & Storage:
| Chemical Name | Iodotrimethylsilane, stabilized with Copper |
| Synonyms | TMIS; TMS Iodide; Trimethyliodosilane; Trimethylsilyl Iodide |
| CAS Number | 16029-98-4 |
| CAT Number | RF-PI2129 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C3H9ISi |
| Molecular Weight | 200.09 |
| Melting Point | <0℃ |
| Boiling Point | 106℃ |
| Specific Gravity (20/20℃) | 1.46 g/mL |
| Sensitive | Light Sensitive, Moisture Sensitive |
| Water Solubility | Reacts |
| Hydrolytic Sensitivity | 8: Reacts Rapidly with Moisture, Water, Protic Solvents |
| Brand | Ruifu Chemical |
Advantages:
FAQ:
Application:
Iodotrimethylsilane (CAS: 16029-98-4) which has a hard acid (Me3Si) -soft base (I) bond displays a strong oxygenophilicity towards ethers, esters, lactones, acetals, and other molecules involving oxygen atom as a functional group. The use of iodotrimethylsilane as the agent brought about a facile cleavage of the tert-butyl ester, giving the corresponding carboxylic acid in excellent yield. Iodotrimethylsilane is used for the introduction of trimethylsilyl group in organic synthesis. It is also useful for gas chromatography analysis by converting alcohol into a silyl ether derivative, thereby making it more volatile than the original molecule. Iodotrimethylsilane is an efficient reagent for ether, ester, carbamate, ketal, and lactone cleavage. For the introduction of the TMS group, e.g., TMS enol ethers. Key reagent for the selective deprotection of an N-Cbz group in the presence of a trimethyltin moiety. Reagent was recently reported to convert allyl- and benzylphosphotriesters to the corresponding iodides. Iodotrimethylsilane is a typical blocking agent in pharmaceutical manufacturing, widely used in the syntheses of drugs. It can protect or deprotect functional groups selectively, act as silane blocking agent. Iodotrimethylsilane is a multipurpose reagent used in various organic reactions. It is used for the dealkylation of few compounds like lactones, ethers, acetals, and carbamates and trimethylsilylating agent for the synthesis of silyl imino esters, alkyl and alkenyl silanes, etc. It also acts as a Lewis acid catalyst and as a reducing agent in many organic reactions. Iodotrimethylsilane can be used as a versatile reagent for the mild dealkylation of ethers, carboxylic esters, lactones, carbamates, acetals, phosphonate and phosphate esters; cleavage of epoxides, cyclopropyl ketones; conversion of vinyl phosphates to vinyl iodides; neutral nucleophilic reagent for halogen exchange reactions, carbonyl and conjugate addition reactions; use as a trimethylsilylating agent for formation of enol ethers, silyl imino esters, and N-silylenamines, alkyl, alkenyl and alkynyl silanes; Lewis acid catalyst for acetal formation, α- alkoxymethylation of ketones, for reactions of acetals with silyl enol ethers and allylsilanes; reducing agent for epoxides, enediones, α-ketols, sulfoxides, and sulfonyl halides; dehydrating agent for oximes.